The role of proline cis/trans isomerization in protein folding and function
The cis/trans isomerization of prolyl peptide bonds is an intrinsically slow reaction and generally rate-limiting for in vitro refolding of proteins harboring cis prolyl peptide bonds in their native, three-dimensional structure. About 6% of the X-Pro peptide bonds in high-resolution X-ray structures of proteins adopt the cis conformation, while cis is only identified in about 0.04% of the non-prolyl peptide bonds. The high frequency of cis X-Pro peptide bonds in protein structures can be explained by the fact that the cis conformer is only about 5 kJ mol−1 less stable than the trans conformer. In chemically denatured proteins, this low energy difference is also responsible for the accumulation of 5–30% non-native cis X-Pro peptide bonds at prolines that are otherwise in trans in the native state (N). This leads to the artificial accumulation of slow-folding molecules in in vitro protein folding studies in which refolding is initiated by rapid dilution from high to low denaturant concentration. This artificial accumulation of slow folding molecules not only complicates the analysis of protein refolding kinetics, but is also often responsible for low refolding yields in the industrial production of recombinant proteins.
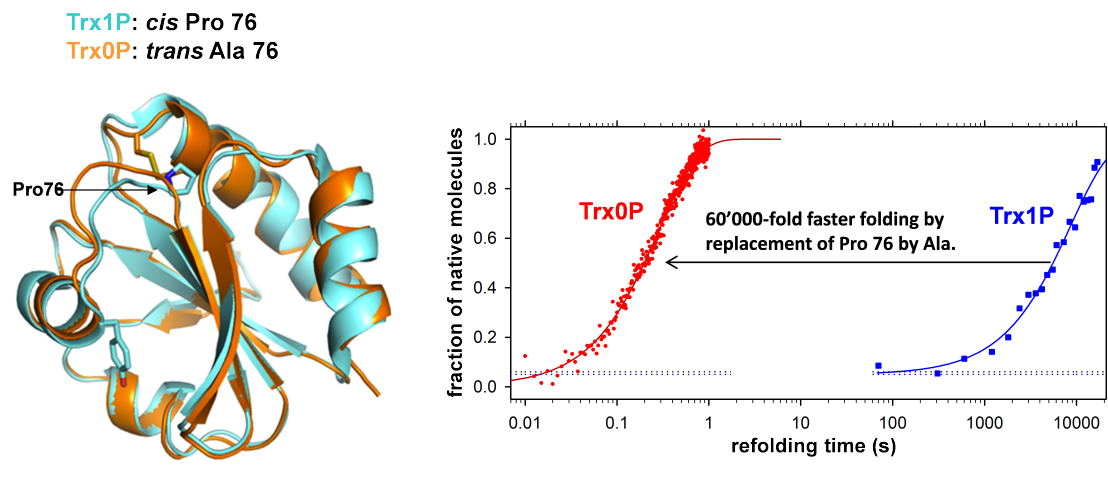
The thioredoxin fold is a classic example of a fold with an invariant cis prolyl peptide bond. To analyze the importance of conserved cis prolyl bonds, we have selected Escherichia coli thioredoxin (Trx) as model system, which is the prototype of oxidoreductases sharing a Cys-Xaa-Xaa-Cys motif in the active site and catalyzing electron transfer reactions via disulfide exchange. The invariant cis-proline (Pro76 in E. coli Trx) is located next to the active-site cysteine pair. To study the role of Pro76 in Trx folding, we created a fully functional, single-cysteine variant, Trx1P, harboring cis-Pro76 as the only proline residue. Interrupted refolding experiments revealed that 95% of the Trx1P molecules only reached the native state extremely slowly (half-life: 100 minutes) due to the population of a kinetic folding intermediate (Itrans) with intact tertiary structure and Pro76 in the nonnative trans conformation. Replacement of Pro76 by Ala yielded a proline-free thioredoxin (Trx0P) which showed perfect two-state behavior in folding, with a folding half-life of 90 milliseconds. The X-ray structure of Trx0P revealed an intact Trx fold with Ala76 in the trans-conformation, in which only minor structural rearrangements in the fold were necessary to accommodate the new trans peptide bond. Our results showed that a single proline-to-alanine substitution can accellerate protein folding by more than four orders of magnitude.
